IMBA's Catarina Martins Costa Earns Vienna BioCenter PhD Award

On November 10, at the Vienna BioCenter PhD Symposium, Catarina Martins Costa was honored with the 2023 PhD Award for her thesis, "From neuroepithelial morphogenesis to circuit formation: using organoid technologies to understand human brain development.” For her PhD research, Catarina focused on understanding the development of the human cerebral cortex and the communication between the two brain hemispheres, mediated by a structure called corpus callosum. Using neural organoids as her primary research tool, Catarina studied the mechanisms of disease leading to the agenesis – or absence – of the corpus callosum in patients carrying ARID1B mutations. Currently available as a preprint, her main project underscores the potential of in vitro models in elucidating key aspects of human brain development that can provide answers to long-standing questions about the biology of the human corpus callosum and associated diseases.
Catarina believes that in vitro modelling of development and disease will revolutionize medicine. She wants to take part in the revolution. In the following interview, Catarina discusses her research, as well as her perspectives on the developing field of biological sciences.
How are you applying stem cell technology in your research?
Pluripotent stem cells are a unique cell type with the remarkable ability to give rise to any tissue found in the human body. In the laboratory, we can culture these cells and expose them to signals that mimic the processes of organ formation, thus directing their differentiation into specific cell types. By clustering a few thousand pluripotent stem cells together in a compact sphere and providing them with signals to mimic brain development, we produce brain organoids: three-dimensional aggregates of various neuronal cell types that closely mimic key aspects of human neurodevelopment. These technologies allow us to better understand how the human brain develops, both in the context of health and of specific diseases encoded in the human DNA.
Could you give us an overview of the findings of your research projects?
In a first project, I studied the effect of the extracellular matrix on the development of brain organoids. The extracellular matrix is the non-cellular component present within tissues, providing structural support to cells. Our work showed that neural progenitor cells in vitro are able to endogenously produce extracellular matrix, undergoing self-organization with minimal exogenous cues. We showed brain organoids can achieve their intended structure without Matrigel, an expensive animal-derived matrix. This was the methodological basis to tackle the central question of my PhD.
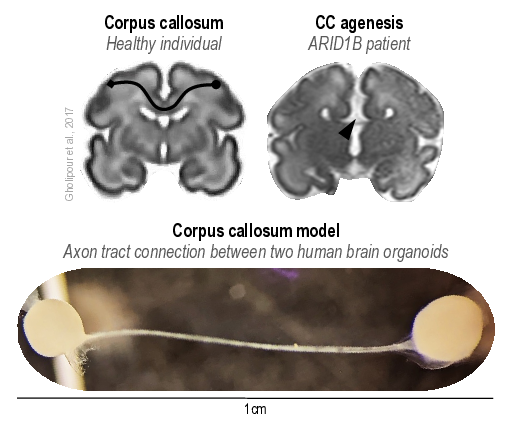
My main project revolved around a fascinating brain structure called the corpus callosum: a bundle of millions of axons that exchange information between the two brain hemispheres. In rare cases, there is a developmental abnormality where this structure is absent, medically referred to as corpus callosum agenesis. Through a collaboration with medical professionals and patients at the Vienna General Hospital, we derived pluripotent stem cells from two patients harboring mutations in the gene ARID1B, responsible for corpus callosum agenesis, and used these cells to generate organoids. Additionally, in partnership with bioengineering experts from the University of Tokyo, we developed the first-ever organoid model of the human corpus callosum. Using this system, we identified defects in callosal neurons, particularly in the formation of long-range axon projections, resulting in corpus callosum agenesis in affected patients.
What excites you most about the future of your field?
In the coming decades, stem cell-based models like organoids will offer unprecedented insights into human biology, overcoming previous limitations in suitable systems. We are moving toward an era where the link between fundamental biology and medicine will be reinforced, particularly at the interface encompassing human development, disease modeling, and drug discovery and testing. These advancements hold great promise for personalized medicine and innovative therapeutic approaches. Looking ahead, I am enthusiastic about all these new experimental avenues and their potential transformative impact on society.
About Catarina
Catarina is a researcher in the Knoblich lab and a recent graduate. She has four first author publications resulting from her doctoral research. She also contributed a book chapter on the evolution and development of the corpus callosum, featured in "Neocortical Neurogenesis in Development and Evolution." In July, Catarina was honored with the City of Vienna Impact Award, one of eight awards given to students from the University of Vienna for the potential societal impact of their research.